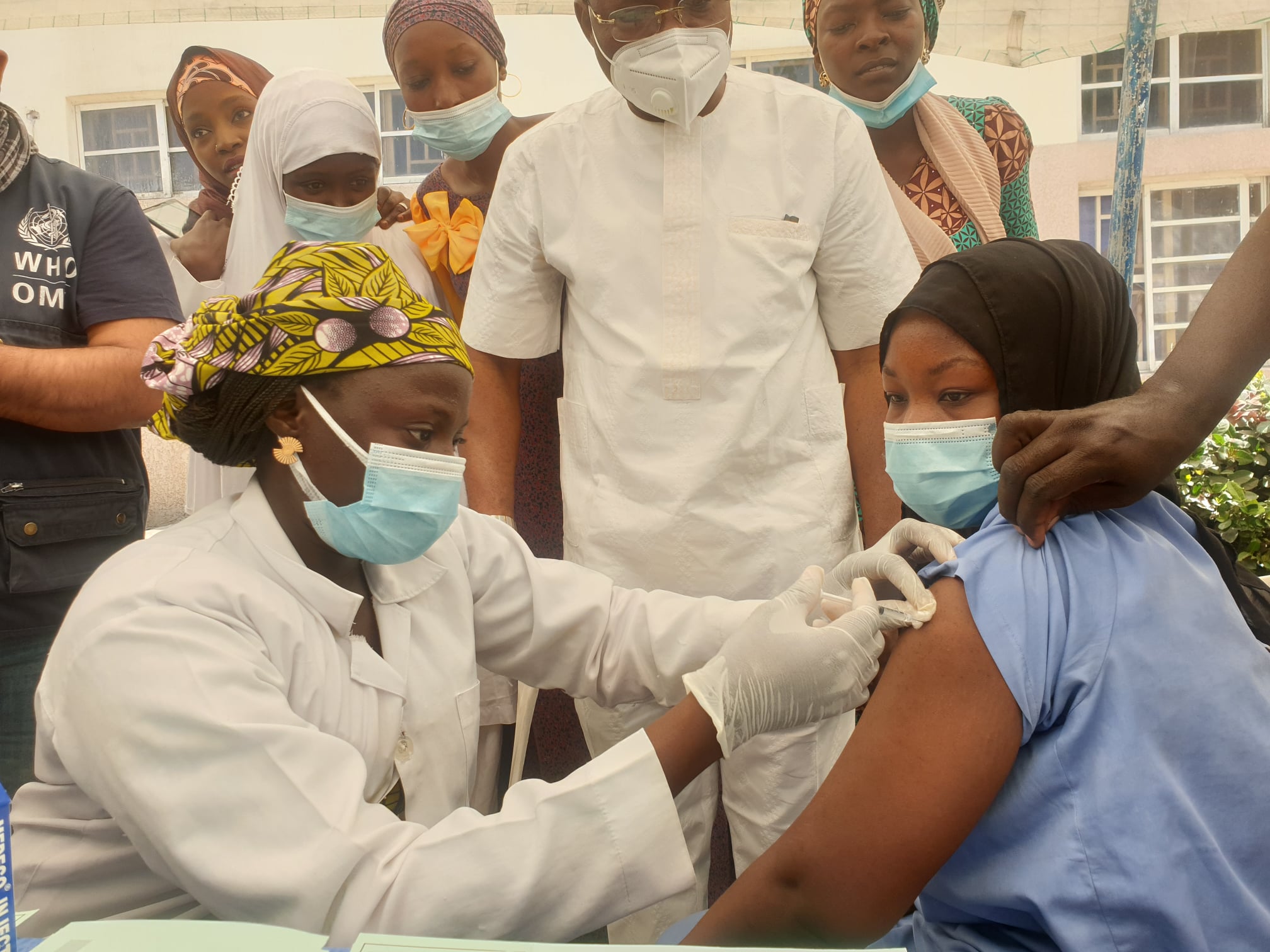
The Federal University Teaching Hospital (FUTH), Lafia, is set to lead the establishment of Nigeria’s first vaccine research and development hub focused on Lassa fever and other infectious outbreaks.
Dr Ikrama Hassan, FUTH’s Chief Medical Director (CMD), unveiled the initiative on Saturday in Abuja during the inaugural vaccine research retreat.
Hassan said that the project was inspired by experience growing up in Lafia, the Nasarawa state capital, where yearly outbreak of a mysterious illness plagued communities, often misdiagnosed as typhoid fever.
“It was not until my time at the University of Ibadan, studying Lassa fever, that I realised what had devastated my community for years was not typhoid.
“Even as a trained physician, I did not know,” he said.
He said that, as a former Director of Health Planning, Research, and Statistics in the state, he initiated the establishment of a vaccine research centre in 2019.
“Despite the facility’s completion, it has remained dormant for over six years. Now, as CMD of the host institution, I have made activating the centre my top priority.
“The impact will be monumental, not just for Nasarawa State, but for Nigeria and Africa as a whole,” he said.
To advance the project, Hassan said that he had brought on board key stakeholders, including Dr Simon Agwale, vaccine development expert and CEO of Innovative Biotech, who also hails from Nasarawa.
He said that Agwale had begun building partnerships with the Africa Centres for Disease Control (Africa CDC) and other global health agencies to support the centre.
“If this group cannot make this centre a reality, I do not think there is another that can,” he said.
He said that the COVID-19 pandemic had underscored the urgency of moving from reliance on imported vaccines to developing local solutions for epidemic-prone diseases like Lassa fever.
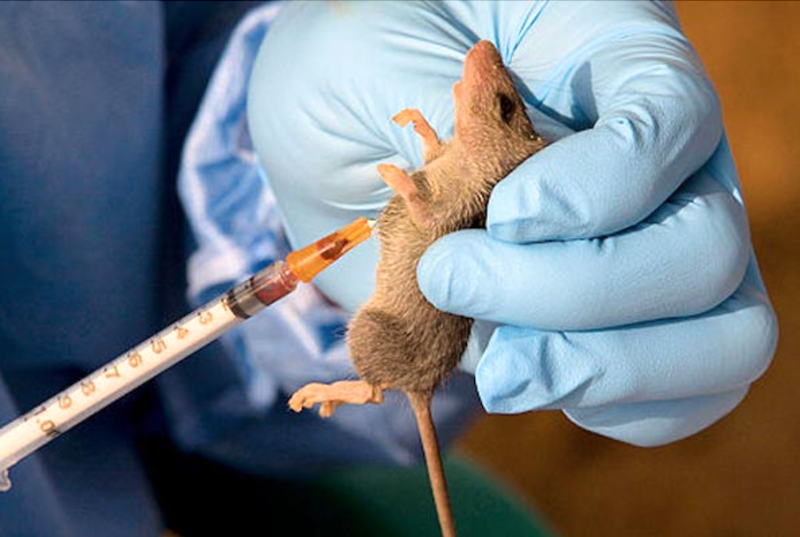
“Lassa fever is endemic in several Nigerian states, including Nasarawa, with annual outbreaks and long-term complications such as hearing loss among survivors.
“Despite its burden on public health, there is currently no widely available Lassa fever vaccine in the country,” he said.
Speaking at the retreat, Agwale highlighted the power of vaccines in saving lives and preventing disease.
He referenced the resurgence of measles in the U.S. as a cautionary tale of vaccine hesitancy.
“Vaccines led to the eradication of smallpox. Today, we talk about eradicating polio only because of vaccines,” he said.
“Vaccine development in Nigeria is hindered by limited early-stage research capacity and the absence of critical infrastructure.
“There is no institution in Nigeria that we can rely on to generate early-stage data for vaccine development,” he said.
He called for urgent investment in animal testing facilities, genomic labs, and other basic research infrastructure.
“Science is not guesswork. Without animal testing infrastructure, it is just guesswork,” he said.
Agwale said that Nigerian biotech companies were often forced to perform roles meant for universities due to capacity gaps in academic institutions.
“As a biotech company, we are doing everything,” he said.
He called for closer collaboration between academia, government, and industry, noting that locally developed vaccines can generate intellectual property (IP) to sustain universities financially.
“If an academic institution develops the vaccine, the IP belongs to them. That’s how research becomes sustainable,” he said.
He also drew attention to the global disparity in health research funding.
“The U.S. National Institutes of Health has a budget over 40 billion dollars, more than Nigeria’s entire national budget,” he noted.