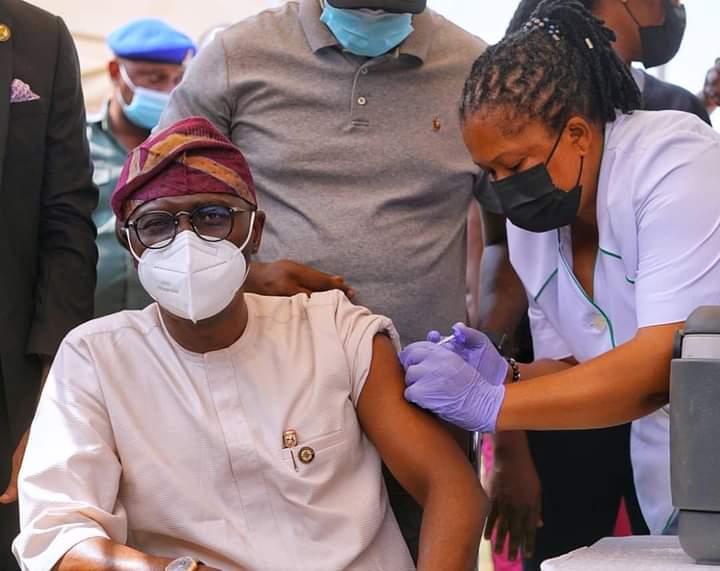
Lagos State Governor, Babajide Sanwo-Olu, on Monday said that he felt no side effects three days after taking the COVID-19 vaccine injection.
Sanwo-Olu said this at Lagos House, Ikeja, while responding to questions from State House Correspondents on his experience after taking the vaccine on March 12.
The governor received the AstraZeneca COVID-19 vaccine alongside the deputy governor, Dr Obafemi Hamzat, the Commissioner for Health, Prof. Akin Abayomi, the Commissioner for Information and Strategy, Mr Gbenga Omotoso.
Also, health practitioners, frontline workers and some newsmen, among others, took the COVID-19 vaccines at the Infectious Disease Hospital (IDH), Yaba,
Sanwo-Olu reassured Lagos residents that the vaccine is safe, hence, they should get vaccinated to curb the spread of the Coronavirus.
”It has been three days since I got the AstraZeneca vaccine. I can confirm to you that I felt no after effects at all. I didn’t have any headache, any malaria or any symptoms whatsoever.
”I have taken and Lagos has continued to vaccinate all of our frontline workers. There is nothing to worry about. We encourage others to continue to take the vaccine,” he said.
Also, the Deputy Governor, Hamzat, said the he felt no after effects.
”No symptoms at all, I didn’t feel anything,” he said.
The Commissioner for Health, Prof. Abayomi, however, said that he felt slight pains at the side of the injection a day after taking the vaccine, but was relieved after taking pain relief medicine.
”I just took some Panadol, I went to sleep, I did a bit of exercise and then I went to sleep and I woke up yesterday morning and I felt fantastic,” he said.
According to him, everybody is different, as some people won’t feel anything at all, some people will have a bit of body pain, some may even have shivering, and those are all expected side effects.
He said that such effects were in the literature of the vaccine and had been mentioned to get people prepared, and such effects were just categorised as `Adverse Effects Following Immunisation’.
”There is another category called `Adverse Effects of Special Interest’ and those are very rare.
“So, the first type of getting pain at the site of the injection, feeling body pain or headache is normal. Most people will experience something like that if you take the vaccine.
”But the one of Special Interest, they are serious, they cause people to collapse, they go into shock, they get severe complications and it can happen with any vaccination, it is not confined to COVID-19.
”They are very rare, they happen; maybe one in five million people who will take it will experience anaphylactic reaction. In other words you react to something in the vaccine, you are allergic to one of the chemical carriers.
”But we are ready for that. If you notice the kits that we have at the site, it contains steroids and all kinds of medications.
“If anybody gets the vaccine and had anaphylactic reaction, the doctors at the site of vaccination are ready to deal with that,” Abayomi said.